|
|
|
|
|
Some services we provide to you
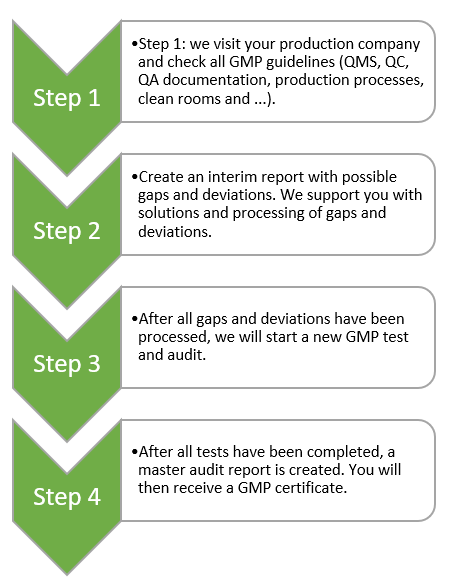
Steps for obtaining GMP certificate
b) How do you get a EU-GMP-License?
If you want to manufacture medicines and/or investigational medicinal products in the European Union or import them into the EU, you need an authorization (EU manufacturing permit) and the corresponding EU GMP certificate. After submitting the respective license application, the supervisory authority carries out an on-site inspection to check compliance with EU good manufacturing practices. We will fully 100% support you in obtaining EU-GMP license. After a successful inspection, the corresponding EU-GMP certificate will be issued. After your application is approved, you will receive an EU manufacturing permit and permission to obtain GMP license. The import of medicines or medical products is therefore permitted. If you are interested in exporting to the EU, please feel free to contact us. We would be happy to advise you. With our GMP expertise, you can meet the GMP requirements for a GMP license within a very short time and gain access to the EU market with 27 countries. |
|